Grove (1919)
What is Coelomycetes mold?
Coelomycetes are an artificially made (form-class) group of conidial fungi (asexual forms) which includes species from Ascomycota and Basidiomycota phyla. These fungi produce asexual spores (conidia) within fruiting bodies called conidiomata, formed in the host’s tissue. Coelomycetes hold species used in biotechnology, bioremediation, and biocontrol, but also many pathogenic fungi. According to estimation, the group of coelomycetes contains about seven thousand species, included in a thousand genera [1].

What are the characteristics of Coelomycetes?
Coelomycetes are ubiquitous, inhabiting mostly tropical and temperate regions and being less common in Antarctic and Arctic regions where fungal diversity is scarce [1]. They mostly exist as saprobes (living on dead or decaying organic matter) in cultivated and non-cultivated soils, dung, debris, and on plants. Some species are symbiotic mycobionts of lichens, and they are called lichenized coelomycetes. Other species are pathogenic, affecting insects, vertebrates, and mammals, including humans [1, 2]. Certain coelomycetous genera are pathogenic to plants and crops, causing leaf, stem, and root lesions and reducing fruit and seed yield [2].
Coelomycetous fungi arise from a vegetative tissue consisting of septate hyphae, which may be glass-like or pigmented, depending on the genus. Most coelomycetous species reproduce exclusively asexually, but some species are linked with sexual stages based on molecular studies [1].
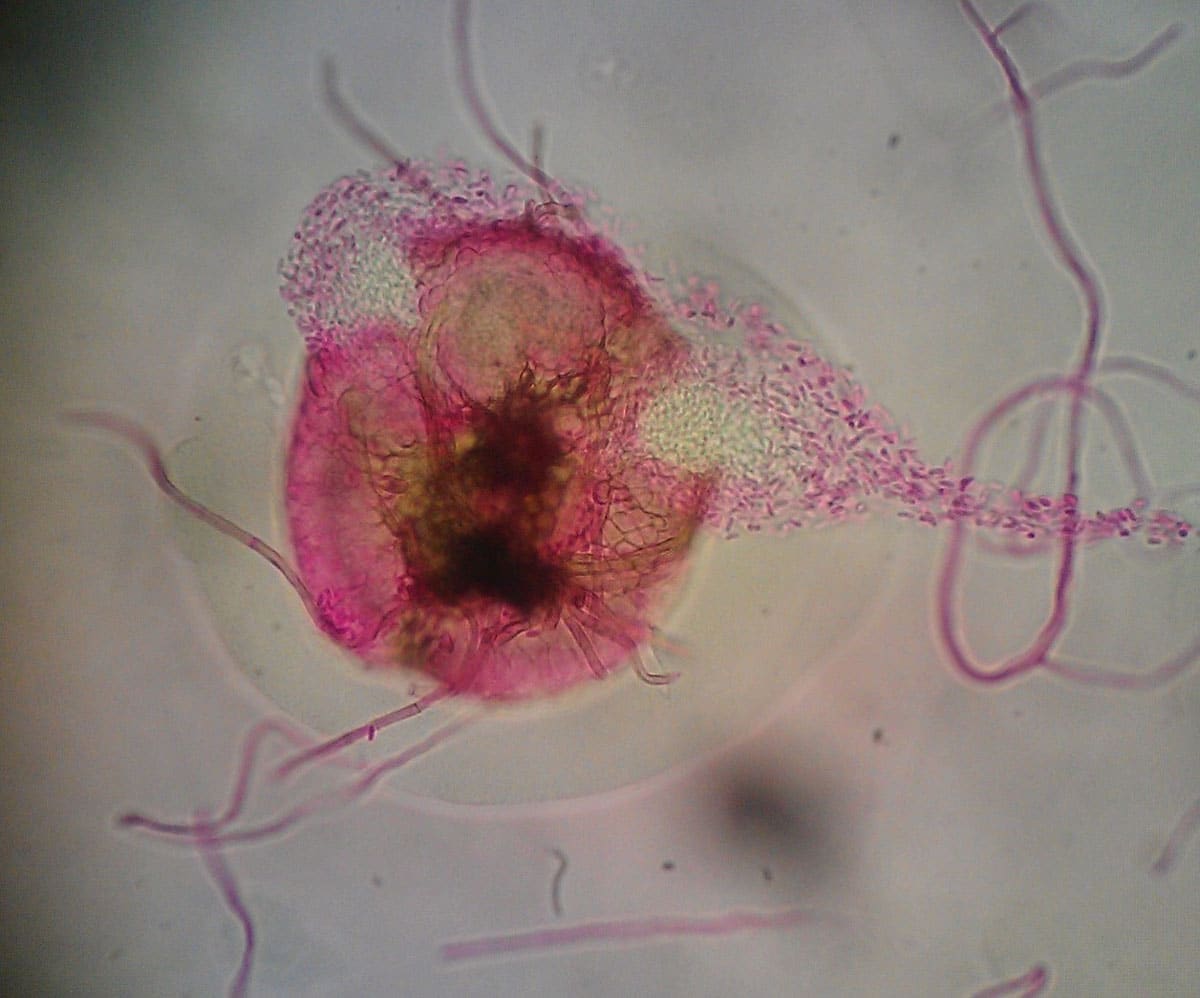
Asexual spores known as conidia are produced within a cavity lined by fungal tissue, host tissue, or a combination of both [1]. There are several types of these fruiting structures called conidiomata (Fig. 1). The most common types of conidiomata are pycnidia, stromata, and acervuli.
Acervuli (Fig. 1) are saucer-shaped structures made up of a mass of conidiophores that develop just beneath the surface of a host plant. As conidia mature, acervuli burst out, pushing aside host tissue as they emerge [3].

Pycnidia (Fig. 2) are spherical structures with an opening, resembling chambers. They can be dark or brightly colored, with a fleshy or tough consistency. Pycnidia can be enclosed with fungal tissue, forming stromata. They may be disc-shaped, spherical, flask-like, or cup-shaped and can be completely closed or open to the air through a hole. Species that produce pycnidia often live on plants as saprobes or pathogens [3].
The infection strategies and the life cycle of coelomycetous fungi
The most common modes of conidial dispersion are by rain splash or by animals (mostly arthropods or insects) [2]. One of the largest and economically most important genera from the group Coelomycetes is Phoma, known as a plant pathogen. The general principle of survival strategies and life cycle can be described using this fungus as an example (Fig. 2).
The main source of infection inoculum of Phoma fungi is diseased plants, containing chlamydospores (thick-walled, large resting spores) and sclerotia (a compact mass of hardened fungal mycelium supplied with food reserves). Certain species also produce pseudosclerotia, solid masses of fungal mycelia mixed with soil. All these structures serve as means of survival during unfavorable conditions [4].
Primary infection originates from overwintering fungal structures (sclerotia, pseudosclerotia, chlamydospores, perithecia, pycnidia) in plant debris, which are spread to non-infected plants by rain splash and irrigation water. Wind and insects can also carry ascospores and conidia. After conidia germinate on the primary infection site, hyphae penetrate the cuticle of leaves and stems. Leaf pathogens usually invade hosts through stomata (openings by which plants regulate the exchange of gases). The pathogen grows intercellularly, colonizing all plant tissues, followed by necrosis. When all plant tissues are successfully invaded, the pathogen produces reproductive structures such as pycnidia or pseudothecia, containing masses of conidia or sexual spores (in species that have sexual stages). They are further disseminated by wind or rain splash. Conidia and mycelial fragments can also be dispersed from the crop debris left in the field after harvest. In the following season, a fresh infection may occur in newly planted crops [4].
Which are known Coelomycetes types?
- Plant pathogenic coelomycetes include several genera, affecting crops, ornamental plants, and timber trees. Some genera have been reported as seed pathogens. Species causing considerable crop and post-harvest losses belong to the genera Ascochyta, Colletotrichum, Diplodia, Dothistroma, Harknessia, Lasiodiplodia, Lecanosticta, Pestalotiopsis, Phaeophleospora, Phoma, Phomopsis, and Phyllosticta [5].
- Endophytic coelomycetes consist of species that inhabit plant organs at some stage in their lives, colonizing internal plant tissues without harming the host. Their function is ecologically important, as they are involved in reducing herbivory, improving the ability of tolerance to unfavorable conditions and diseases, and decomposing [5].
- Lichen-forming coelomycetes include ascomycetes that produce both sexual and asexual stages on the same thallus and enter a symbiosis with algae to form lichens [5].
- Fungicolous coelomycetes include species that grow on other fungi as parasites or saprobes [5].
- Mycorrhizal coelomycetes inhabit the root systems of trees, making mutual symbiotic associations with their hosts [5].
- Coelomycetous fungi infecting humans belong to several genera of pycnidial coelomycetes, occasionally isolated in cases of the human subcutaneous disease, keratitis, and deep tissue infection. However, documentation about infections caused by coelomycetes is scarce due to a lack of adequate identification tools. Genera linked to human diseases are Coniothyrium, Paraconiothyrium, Microsphaeropsis, Nattrass, Phoma, Pleurophoma, Colletotrichum, and several others [6].

Did you know?
The #1 toxic mold type found in homes is the Penicillium/Aspergillus mold group?! Find out more exciting mold stats and facts inside our mold statistics page.
References
- Wijayawardene, N. N. et al. (2012). Coelomycetes. Cryptogamie, Mycologie, 33(3):215-244
- Stchigel A. M. & Sutton D. (2013). Coelomycete Fungi in the Clinical Lab. Clinical Lab Issues. 7:171–191
- Moore, D. et al. (2021). 21st Century Guidebook to Fungi. Retreated from: www.davidmoore.org.uk
- Deb, D. et al. (2020). Phoma diseases: Epidemiology and Control. Plant Pathology. 69 (7): 1-15
- Wijayawardene, N. N. et al. (2016). Taxonomy and phylogeny of dematiaceous coelomycetes. Fungal Diversity. 77:1–316
- Stchigel, A. M. & Sutton, D. A. (2013). Coelomycete Fungi in the Clinical Lab. Current Fungal Infection 7:171–191

Get Special Gift: Industry-Standard Mold Removal Guidelines
Download the industry-standard guidelines that Mold Busters use in their own mold removal services, including news, tips and special offers:

Written by:
Jelena Somborski
Mycologist
Mold Busters
Edited by:
Dusan Sadikovic
Mycologist – MSc, PhD
Mold Busters
